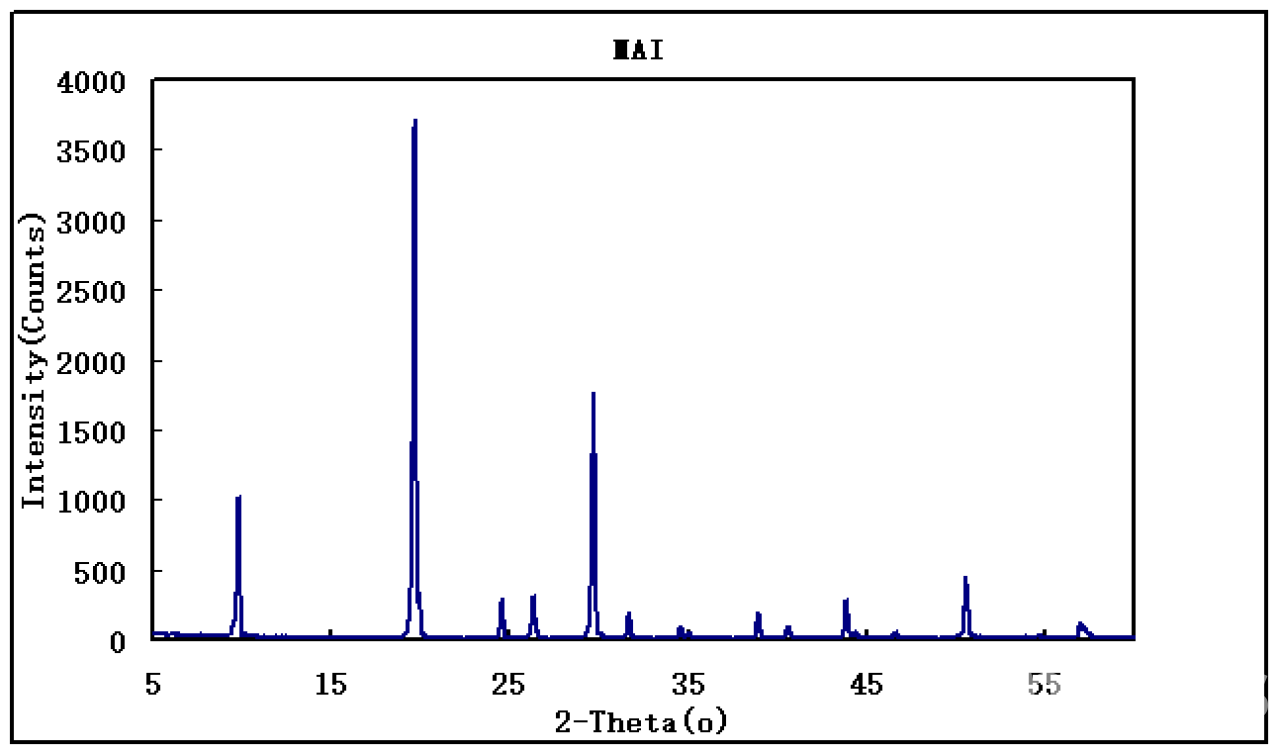
| Notes | XRD & 1HNMR spectra, user instruction for enquiring |
| Store | 25℃ under N2 atmosphere |
| Packaging | 10 g, or as required in glass bottle |
| Solubility | High soluble in EtOH, DMF, DMSO, water et al |
| Appearance | White to beige solid |
| Purity | 99.5% |
| Linear Formula | CH6IN |
| CAS Number | 14965-49-2 |
| Mol. Weight | 158.97 |
| Name(EN) | MAI |
| Synonym | CH3NH3I/MAI |
| Stock Name | MAI |
A study on the effects of mixed organic cations on the structure and properties in lead halide perovskites
[Physical Chemistry Chemical Physics, 2020, vol. 22, # 5, p. 3105
Abstract
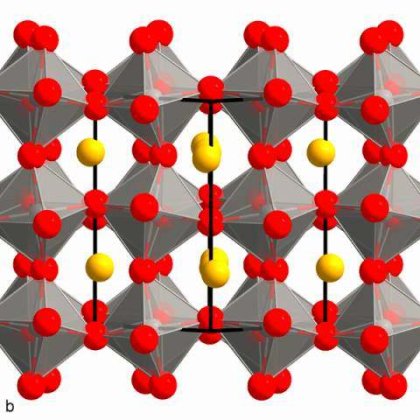
Recently, organic-lead halide perovskites have emerged as strong competitors in photovoltaic and general optoelectronic applications owing to their remarkable characteristics, including high balance hole and electron mobility, strong absorption coefficient and long carrier lifetime. However, the commercial applicability of these materials is hampered by their relative lack of stability compared to established inorganic and organic semiconductors. It has been found that it is possible to tune the properties and stability of the organic-lead halide perovskite materials by site-substitution at A sites of the ABX3 perovskite structure. Here, organic cations (NH4+, HC (NH2)2+, and CH3CH2NH3+) were successfully incorporated in the methylammonium-based perovskite crystal to investigate the role of organic cation size on structure, optical features, thermal stability, and electrical transport properties. Powder X-ray diffraction results indicate that the size of organic cations can not only cause lattice strain by lattice contraction or dilation but also may induce phase transitions by octahedral tilting. Meanwhile, band gaps of these crystals show that organic cations could tune the band gap energy of the perovskites by changing the Pb-I bond angle, which agrees with previous reports. The result of thermogravimetric analysis indicates that thermal stability is related to the probability of HI formation, which is directly related to the acidity of the organic species. These results represent an important step to highlight the role of organic cations in hybrid perovskite materials, which will further benefit the fundamental understanding of materials and device optimization.
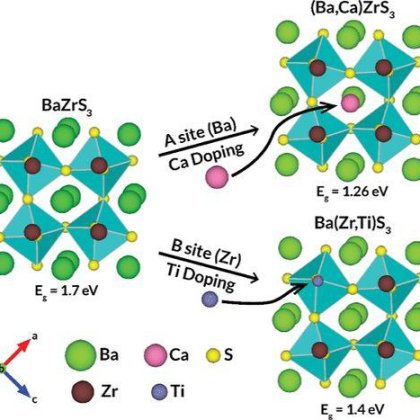
Ba2+ Doped CH3NH3PbI3 to Tune the Energy State and Improve the Performance of Perovskite Solar Cells
[Electrochimica Acta, 2017, vol. 254, p. 165 - 171]Abstract
Elements substitution and doping in perovskite CH3NH3PbI3 exhibit versatile tunability of energy band structure and opto-electric properties. Ba2+ is chosen to substitute Pb2+ for its similar valence state and ionic radius with Pb2+. Ba2+ doping in perovskite (mol% <5) slightly enlarges the optic energy gap by conduction band minimum(CBM) upshifting to vacuum energy level, which is due to the smaller electronegativity of Ba than Pb. The enlarged band gap is also verified by density function theory calculations. In n-i-p structure perovskite solar cells (PSCs), because of the higher CBM of doped perovskite, the Fermi energy difference between n and p side is enlarged and the electron injection from the perovskite to TiO2 is improved. Thus, both the photovoltage and photocurrent are improved by small amount Ba2+ doping, resulting optimized 17.4% efficiency under AM1.5. This work reveals the relationship between the doping element property and the energy band structure of the perovskite, and highlights the doping method to improve the performance of PSCs.
Subphthalocyanine as hole transporting material for perovskite solar cells
[RSC Advances, 2015, vol. 5, # 85, p. 69813 - 69818]Abstract
Non planar 14-π aromatic subphthalocyanine has been introduced for the first time as hole transporting material for organometal halide perovskite solar cells and achieved a power conversion efficiency of 6.6%. Cells stored in the dark under ambient conditions underwent an incubation period of nine days during which, we observed an increase in efficiency followed by slow progressive deterioration. However, Raman spectral analysis of pristine perovskite deposited on titania revealed a much faster degradation thus indicating that the subphthalocyanine layer provides a temporary protection to the underlying perovskite layer.
| Characteristic 1 | I |
| Characteristic 2 | NH3 |