| Notes | 1HNMR & user instruction for enquiring |
| Store | 25℃ under N2 atmosphere |
| Packaging | 5 g, or as required in glass bottle |
| Solubility | High soluble in DMF,DMSO, EtOH et al. |
| Appearance | White/beige solid |
| Purity | 99.5% |
| Linear Formula | C8H12ClN |
| CAS Number |
156-28-5 |
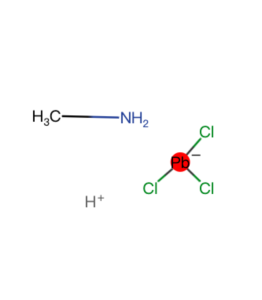
| Synonym | C6H5CH2CH2NH3Cl/PEACl |
| Stock Name | PEACl |
| Mol. Weight | 157.64 |
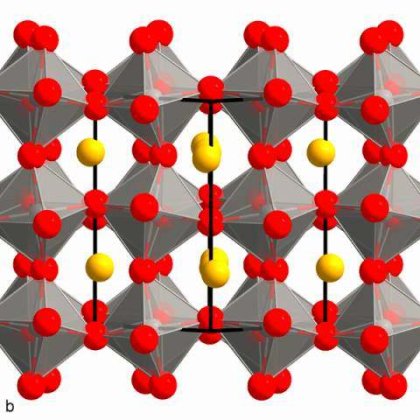
Metal halide perovskites are considered the most promising candidates for solar cells of the decade due to their exceptional optical and electronic properties. The power conversion efficiency of metal halide perovskites, when incorporated as the active layer of solar cells, has become comparable to that observed for conventional silicon solar cells. However, the stability, scaleup, green solvent usage, and fabrication in ambient conditions of metal halide perovskites need to be solved for commercial applications. Here, we report the fabrication of blade-coated methylammonium lead iodide (MAPbI3) perovskite thin films using methylamine and acetonitrile as “green” solvents under ambient conditions. Our perovskite films are initially prepared from low purity PbI2 (99%) and are blade-coated in dry air at relative humidity (RH) levels above 30%. A significant advantage of fabricating our perovskite thin films via blade-coating protocols is that there is a minimal amount of precursors (5 μL) used compared to spin-coating methods (50μL–60μL) for a 4 cm2 substrate. With the addition of a small amount of an organic halide salt, namely, phenethylammonium chloride, the film crystallinity is improved and non-radiative recombination is suppressed, resulting in power conversion efficiencies over 20%. In addition, the device maintains more than 95% of its initial efficiency after 500 h under continuous light illumination of 1-sun at open circuit conditions, 50 °C and 60% RH. The above method leads a path towards the commercial fabrication of perovskite solar cells.
| Characteristic 1 | Cl |
| Characteristic 2 | NH3 |